Great! Now we have the water effectively removed from the product. Are we done?
Well, no. Conceptually, we are done, but there are many things we need to fix realistically.
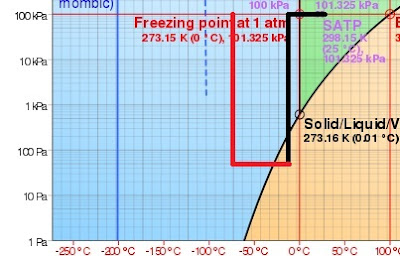
Just the process of drawing a vacuum can be complicated. One of the issues is what to do with the cold water vapor. See, as the cold water vapor exits the vacuum pump, it follows the same black line as the product followed, except in reverse.
It is not important to understand the complexities of a vacuum pump, but just realize that somewhere in that pump, the pressure changes from a vacuum, to atmospheric pressure.
The water vapor starts at the bottom left point on the line. As it travels through the pump, it will eventually shoot up to the top of that vertical line. Notice that this changes from water vapor to ice. This absolutely means the pump will freeze up.
Notice that the vapor will turn to ice if we increase the pressure. But also notice it will turn to ice if it gets colder, too. This is important! We can move the vapor through a much colder area and convert it to ice. If we convert all of the water to ice at operational pressures, there will be no water vapor exiting the vacuum pump. We have trapped it in the cold trap.
With the cold trap in place, the water vapor follows the red path instead of the black path. The only issue is that we need to maintain 2 different temperatures, one for the product and one (much colder) for the cold trap.
Notice that the red line does not move upward until the water is converted to ice. So the air exiting the vacuum pump will be dry. For that reason, no ice can form in the vacuum pump.

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.